Drosophila models of aging and Human disease
Aging is a universal but poorly understood biological process that involves a complex interplay between environmental and genetic factors. Model organisms, such as Drosophila, have provided an opportunity to search for and identify specific genes that affect lifespan. We have previously found that increased expression of SOD1 or Mgat1 in the neurons of normal flies increases their lifespan by up to 40% and 135% respectively. These exciting results demonstrate that single genes acting in specific cells such as neurons, can have a profound effect on the lifespan of an organism. More recently, we have also found that the immune system plays an important role in regulating lifespan and our current efforts are aimed at understanding the mechanisms involved.
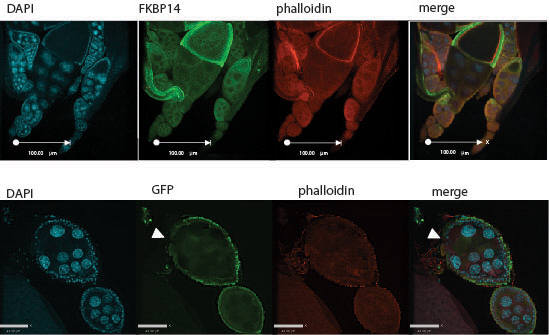
We have also used Drosophila to study the molecular and cellular mechanisms underlying the pathogenesis of human disease. Initially, we focussed on understanding the role of presenilins, which were first identified as causative factors in familial Alzheimer's disease, but have since been shown to play a critical role in Notch signalling during development. We showed that presenilin is required for neuronal differentiation and that it affects Notch subcellular localization and signalling. We have also shown defects in both synaptic strength and plasticity in presenilin null mutants, and defects in associative learning indicating an important role for presenilin in normal neuronal function. To further understand the role of presenilin, we conducted a genetic screen to identify novel genes capable of modifying the function of presenilin. One of these, calmodulin, is a calcium binding protein that interacts with presenilin and affects intracellular calcium homeostasis. Another, FKBP14, regulates presenilin protein levels and activity. Current efforts are focussed on characterizing these and other modifiers identified in our screen.
We have also recently developed Drosophila models to study obesity and its regulated disorders. Specifically, we are interested in understanding how the CNS regulates different behaviours (e.g. feeding behaviours) and the metabolic state of flies, and how these activities are co-ordinated with changes in environmental influences (e.g. the availability of food). To this end, we have performed a wide-scale RNAi screen to identify genes that act in the nervous system to regulate fat storage in flies. Current efforts are aimed at understanding the function of genes identified in the screen.
Key References:
Trihn, I., Gluscenove, O. & Boulianne, G.L. (2013). Modeling obesity and its associated disorders in Drosophila. Physiology 28, 117-124.
Sarkar, M., Iliadi, K.G., Leventis, P.A., Schachter, H., and Boulianne, G.L. (2010). Neuronal expression of Mgat1 rescues the shortened life span of Drosophila Mgat11 null mutants and increases life span. Proc Natl Acad Sci U S A 107, 9677-9682.
Michno, K., Knight, D., Campusano, J.M., Van De Hoef, D., and Boulianne, G.L. (2009). Intracellular calcium deficits in Drosophila cholinergic neurons expressing wild type or FAD-mutant presenilin. PLoS ONE 4, e6904.
van De Hoef, D.L., Hughes, J., Livne-Bar, I., Garza, D., Konsolaki, M., and Boulianne, G.L. (2009). Identifying genes that interact with Drosophila presenilin and amyloid precursor protein. Genesis 47, 246-260.
Sakar, M., Leventis, P.A., Silvescu, C., Reinhold, V.N., Schacter, H. & Boulianne, G.L. (2006). Null mutations in the Drosophila N-acetylglucosaminyltransferase I produce defects in locomotion and a reduced lifespan. J Biol Chem 281, 12776-12785.
Knight, D., Iliadi, K.G., Charlton, M.P., Atwood, H.L. & Boulianne G.L. (2006). Presynaptic plasticity and associative learning are impaired in a Drosophila presenilin null mutant. Dev Neurobiol 2007, 1598-1613.
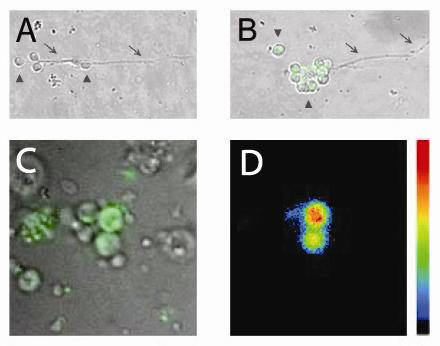
For more detail, see Michno et al, 2009
Research
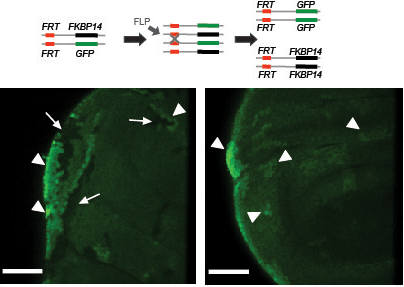
FKBP14 is required for cell viability. Mutant clones were induced in imaginal discs using FLP-recombinase mediated site-specific recombination (see schematic). In wild-type discs (left), GFP negative clones are observed adjacent to homozygous GFP twin spots (visible as brighter GFP staining). In FKBP14 mutant clones however, the GFP double positive twin spots are apparent, but the GFP negative FKBP14 mutant clones are absent indicating a loss of the FKBP14 mutant cells.
For more detail, see
van de Hoef etal 2013
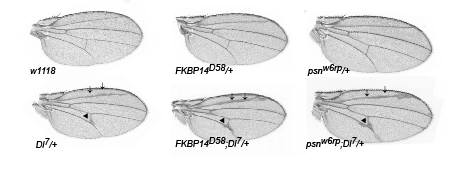
FK506 Binding Protein 14 (FKBP14) was identified in a genetic screen for modifiers of presenilin function. Delta is a Notch ligand, and mutations in delta lead to characteristic defects in wing veins (bottom left). Preseniln (psn) is core component of the
g
-secretase complex is is known to genetically interact with the Notch signalling pathway. Mutations in psn enhance these wing vein phenotypes (bottom right wing). Interestingly, mutations in FKBP14 similarly enhance the delta wing phenotype, suggesting that FKBP14 also genetically interacts with the Notch signalling pathway and we have recently shown that FKBP14 affects presenilin protein levels.
Image courtesy of Maeve Bonner