

Synaptic plasticity and behaviour
Fast and efficient synaptic transmission depends on mechanisms that regulate both the release (exocytosis) of synaptic vesicles at the nerve terminal and their recycling (endocytosis). We have focused on understanding the mechanisms that regulate both processes in vivo. Using molecular and biochemical approaches, we were able to identify novel proteins and determine their role in synaptic vesicle exocytosis. We have also shown that many of these molecules can regulate additional membrane trafficking events within the cell and are required for Notch-dependent signaling during development. Importantly, we have shown that regulating the levels of specific proteins, known as SNARES, alters the ability of neurons to sense calcium, which is essential for neurotransmitter release and all brain function including learning and memory (Stewart et al 2000). We have also used genetic approaches to identify additional genes that regulate synaptic plasticity and behaviour. We found that one of these genes, nemy (no extended memory), plays and essential role in memory formation by regulating neuropeptide amidation (Iliadi et al, 2008). We also identified mutations in ent2 (equilibrative nucleoside transporter 2) and showed that it regulates synaptic plasticity, learning and memory and ethanol sinsitivity by altering adenosine signalling at synapses (Knight et al, 2010). Current efforts are aimed at understaning the role of neuromodulator octopamine and the neural adhesion molecule neuroligin in both synaptic function and behaviour.
Key References
:
Knight, D., Harvey, P.J., Iliadi, K.G., Klose, M.K., Iliadi, N., Dolezelova, E., Charlton, M.P., Zurovec, M., and Boulianne, G.L. (2010). Equilibrative nucleoside transporter 2 regulates associative learning and synaptic function in Drosophila. J Neurosci 30, 5047-5057.
Iliadi, K.G., Avivi, A., Iliadi, N.N., Knight, D., Korol, A.B., Nevo, E., Taylor, P., Moran, M.F., Kamyshev, N.G., and Boulianne, G.L. (2008). nemy encodes a cytochrome b561 that is required for Drosophila learning and memory. Proc Natl Acad Sci U S A 105, 19986-19991.
Stewart, B.A., Mohtashami, M., Trimble, W.S. & Boulianne, G.L. (2000). SNARE Proteins contribute to calcium cooperativity of synaptic transmission. PNAS 97,13955-13960.
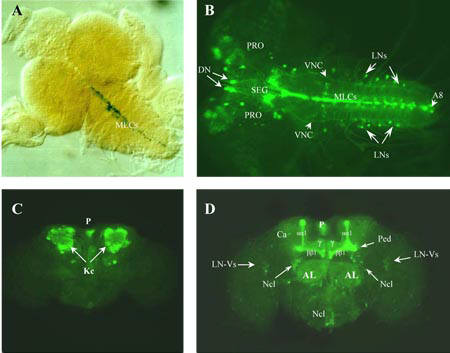
For more detail, see Iliadi et al. (2008)
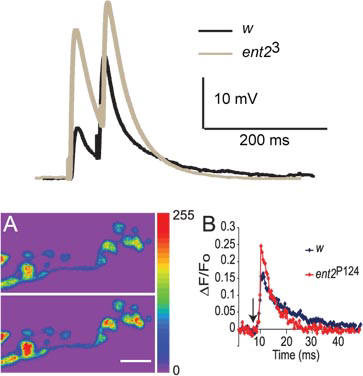
For more detail, see Knight et al. (2010)
Research
We are currently using the Noldus Ethovision XT Video tracking software to record and analyse fly behaviour. One interesting novel behaviour we have recently observed is "fainting" flies. We recently noticed this novel behaviour in a mutant we have been studying. When mechanically startled, wild-type flies show little change in the behaviour. The mutants however, appear to faint for a few seconds, before resuming normal activity. We are currently invesitgating the underlying neural pathways involved in this behaviour. To see the fainting flies, click the you-tube link to the left.