Williams-Beuren Syndrome


Williams-Beuren syndrome
Genotype-Phenotype Correlation in WBS
Genomic Rearrangements of the WBS Region
Williams-Beuren syndrome (WBS)(OMIM #194050) is caused by the hemizygous deletion of a 1.5 Mb region of chromosome 7q11.23. WBS presents with a wide spectrum of clinical symptoms affecting almost every body system. These include cardiovascular lesions, recognizable facies, dental problems, hypersensitivity to specific sounds, hypercalcemia, glucose intolerance and musculoskeletal abnormalities. Individuals with WBS also have a complex cognitive and behavioural profile which includes mild mental retardation, relative strength in language, deficits in spatial processing, and attention deficit hyperactivity disorder, along with a unique combination of overfriendliness and anxiety.
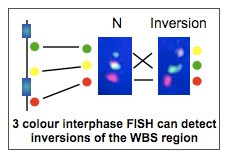
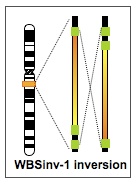
Because of the genomic structure of 7q11.23, the region is also prone to inversion. These inversions make the chromosome more likely to undergo additional rearrangements in the next generation, and so are associated with a higher risk of deletion or duplication. This inversion is present in the general population as a common polymorphism (6% of people carry one chromosome with a 7q11.23 inversion) and does not result in clinical features.
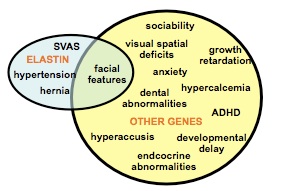
WBS is caused by the deletion of between 26 and 28 genes but to date Elastin, which lies near the centre of the common deletion, is the only gene unequivocally linked to a specific clinical symptom – cardiovascular disease. The specific role of the remaining genes is unknown.
Collaborators:
Matthew Hurles (Sanger Centre, Cambridge)
Our current research is aimed at learning more about the effect of the inversion on meiotic recombination. We are doing this by looking at sperm from carriers of the inversion to asses the nature and quantity of non-allelic homologous recombination in the germ cells. This study is actively recruiting participants. Please contact Lucy Osborne for further information and see the Clinical Research Studies page for the appropriate family information sheet and consent forms.
Our current research is aimed at working out which genes are contributing to the clinical phenotype of WBS through the study of individuals with smaller or larger deletions of 7q11.23. Please contact Lucy Osborne for further information and see the Clinical Research Studies page for the appropriate family information sheet and consent forms.
The genes that cause most of the features of WBS are still unknown since the vast majority of patients carry the same sized deletion. Patients with unusual genomic rearrangements of 7q11.23, such as smaller deletions, are therefore a valuable tool for dissecting the genotype/phenotype relationship in WBS. We use fluorescence in situ hybridization (FISH) analysis of patient lymphoblastoid cell lines and quantitative real-time PCR analysis to define the extent of the deletions in both typical and atypical patients.
Collaborators:
Colleen Morris (University of Nevada)
Carolyn Mervis (University of Louisville)
Barbara Pober (Massachusetts General Hospital, Boston)
We have turned to the mouse in order to achieve our aim of understanding the role of specific genes in the WBS phenotype. We have generated single gene and transgenic mutants to further investigate the function of individual candidate genes, and are also studying the combinatorial effects of gene deletion through the analysis of deletion mouse models. These mice will enable us to study developmental time-points and tissues not accessible in humans and should allow the identification of some of the primary genetic pathways that are altered in WBS.
Animal Models of WBS
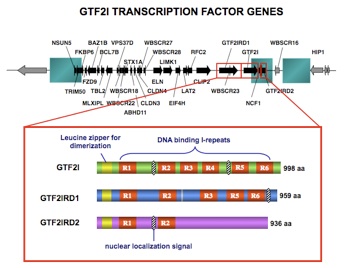
Our focus is on Gtf2ird1 and Gtf2i, transcription factor genes that are linked to many of the WBS cognitive and behavioural symptoms. A mouse knock-out of Gtf2ird1 exhibits increased social interaction and impaired innate fear and current research is aimed at probing the underlying molecular and physiological basis for this phenotype.
Collaborators:
Evelyn Lambe (University of Toronto)
John Roder (Samuel Lunenfeld Research Institute, Toronto)
Herbert Gaisano (University of Toronto)
A transgenic mouse model over-expressing one of the genes from within the WBS deletion, Syntaxin 1A, demonstrated impaired glucose tolerance and insulin secretion, suggesting it may play a role in diabetes and glucose intolerance in people with WBS. We are currently studing the role of STX1A in insulin secretion and diabetes using a Stx1a beta-cell specific knock-out mouse.
