Pediatric Annals July 1998; 27 (7):
445-455.
Vaccine Safety:
Current and Future
Challenges
Robert T. Chen MD, MA
Chief, Vaccine Safety and
Development Activity
Beth Hibbs RN, MPH
National Immunization Program (E-61)
Centers for Disease
Control and Prevention (CDC)
Atlanta, GA 30333
Educational Objectives
1. Discuss the importance of vaccine safety research.
2. Review how vaccine safety is monitored as well as current gaps and limitations in our knowledge in the field of vaccine safety.
3. Discuss the vaccine injury compensation program, risk communication, and helpful resources for specific questions about vaccine safety. |
Introduction
Immunizations are responsible for preventing death and disability
from disease and are among the most cost-effective and widely used public health
interventions. However, it is also recognized that no vaccine is perfectly safe
or effective. Some people will experience side effects from vaccines, and a few
may not experience a complete immunologic response to a vaccine, leaving them
susceptible to disease. Sustaining high vaccine coverage rates may become more
of a challenge as people question the limitations of vaccines and new parents no
longer see or hear about the diseases from which vaccines protect them. In the
future, maintaining public support for immunizations will be critical for
preventing outbreaks of vaccine preventable diseases. In this chapter, we will
focus on the importance of vaccine safety research; current gaps and limitations
in knowledge in the field of vaccine safety; how vaccine safety is monitored;
the vaccine injury compensation program; vaccine risk communication, and helpful
resources for specific questions about vaccine safety.
The Importance of Ongoing Vaccine Safety Research
Vaccine adverse event reports include health events caused by vaccines (i.e.,
true adverse reactions) and those that are not caused by vaccines (associated
only by coincidence). In maturing immunization programs,
(figure 1) close monitoring and timely assessment of suspected vaccine
adverse events are critical to prevent loss of confidence, decreased vaccine
coverage, and return of epidemic disease. 1,2 This has been
experienced in several countries for pertussis,3 and more recently,
diphtheria. 4
 Figure 1. Evolution of immunization program and prominence of vaccine safety.
Figure 1. Evolution of immunization program and prominence of vaccine safety.
There are several key considerations for supporting and maintaining an active
vaccine safety program. First, a higher standard of safety is generally expected
of vaccines than of other medical interventions because, in contrast to most
pharmaceutical products, which are administered to ill people for curative
purposes, vaccines are generally given to healthy people to prevent disease.
Public tolerance of adverse reactions related to products given to healthy
people, especially healthy babies, is substantially lower than to products
administered to people who are already sick.5 This lower risk
tolerance by the public for vaccines translates into a need to investigate rarer
possible causes of adverse events following vaccinations than would be
acceptable for most other pharmaceutical products.
Another consideration is based on the widespread use of vaccines and the
potential extensive impact of problems or concerns to develop in a large
percentage of the population that receives them.6 An example of this
potential impact can been seen in recent concerns that polio vaccine
contaminated by simian virus 40 may have been received by millions of people
during the 1950's.7 Another concern recently expressed in Britain was
the possibility that vaccines may have contained gelatin stabilizers produced in
cattle infected with bovine spongiform encephalopathy. 8 The
importance of ensuring the safety of a relatively universal human-directed
"exposure" like immunizations is the basis for strict regulatory control of
vaccines by the Food and Drug Administration (FDA) in this country.9
A high standard for vaccine safety is also needed, because providing a record of
immunization is mandated under many state and local school entry requirements.
Unlike many classes of drugs for which other effective therapy may be
substituted, vaccines generally have few alternative strains or types to chose
from. The decision to withdraw a vaccine or switch between strains may also have
wide ramifications. For example, the withdrawal of the 1976 "swine influenza"
vaccine due to elevated risk of Guillain-Barré syndrome led to many lawsuits and
low public acceptance for influenza vaccinations for a decade.10
Establishing associations with vaccines and promptly defining the attributable
risks are critical in placing adverse events in the proper risk-benefit
perspective. An erroneous association or attributable risk can undermine
confidence in a vaccine and have disastrous consequences for vaccine acceptance
and disease incidence. On the other hand, denials of association despite
accumulating evidence can also undermine public confidence. 11,12
Research in vaccine safety can help to distinguish true vaccine reactions
from coincidental unrelated events and may help to maintain public confidence in
immunizations and the credibility of immunization programs.
Limitations and Gaps in the Science of Vaccine Safety
In 1967, the lack of scientific documentation on the hazards of immunization
moved Sir Graham Wilson, former director of the Public Health Laboratory Service
in the U.K., to compile the first vaccine safety review. He noted fear of
compensation claims or inadvertent support to "anti-vaccinationaists" as
possible explanations for the incomplete records.5
In the United States, The National Childhood Vaccine Injury Act of 1986
established a Committee from the Institute of Medicine (IOM) to review the
adverse consequences of childhood vaccines. This group found severe limits in
the knowledge and research capability on vaccine safety. Of the 76 vaccine
adverse events they reviewed for causal relation, 50 (66%) had no or inadequate
research. Specifically, the IOM Committees identified the
following limitation's: 1) Inadequate understanding of
biologic mechanisms underlying adverse events; 2) Insufficient or inconsistent
information from case reports and case series; 3) Inadequate size or length of
follow-up of many population-based epidemiologic studies; 4) Limitations of
existing surveillance systems to provide persuasive evidence of causation and 5)
Few experimental studies published relative to the total number of epidemiologic
studies published.13,14
Vaccine safety research requires expertise in "rare disease"
epidemiology.15,16 Such studies are costly and difficult to organize
and may be less familiar to most immunization experts with mainly an infectious
disease background. Like other areas of safety (e.g., food), vaccine safety
cannot be studied directly. Typically, vaccine safety studies that are
epidemiologically based are inferred by the sum of the inverse: an absence of
specific problems when appropriate surveillance and risk management systems are
in place. Scientifically, it is more challenging to prove than disprove a
concept, especially a negative concept.17
The IOM concluded that "if research capacity and accomplishments [are] not
improved, future reviews of vaccine safety [will be] similarly handicapped."
Although much remains to be done, much progress has been made the past few years
toward understanding these gaps and ameliorating the research infrastructure to
improve vaccine safety. 1,2,18-22
Methods of
Monitoring Vaccine Safety
The importance of ensuring vaccine safety encompasses a broad range of
activities. Pre-licensure evaluations and post-licensure surveillance systems
are in place at the federal level. Safety issues regarding the storage,
handling, and recording of vaccines by individual clinicians are also an
important component in considerations in vaccine safety.
Pre-licensure
Like other pharmaceutical products, vaccines, undergo extensive safety and
efficacy evaluations in the laboratory, in animals and in sequentially phased
human clinical trials prior to licensure.9 Phase I
trials usually number in the tens and can only detect the grossest toxicity.
Phase II trials generally enroll hundreds of people and, when
carefully coordinated, can provide important conclusions. These conclusions
might address the relationship between the concentration of antigens, number of
vaccine components, formulation technique, effect of successive dose and profile
of common reactions, all of which impact on the choice of the vaccines chosen
for Phase III trial.23,24 The sample size for Phase
III vaccine trials are based primarily on efficacy considerations,
usually ranging between 100 and 10,000 participants. The maximum duration of
observation is also generally limited to a couple of years. The availability of
unvaccinated control group, however, allows clear identification of true common
local and systemic reactions (e.g., injection site swelling, fever,
fussiness).23 To identify potential safety problems, vaccines go
through pre-release lot testing for safety and potency.9,25 This
evaluation usually occurs parallel to the clinical trials prior to vaccine
licensure.
Post-licensure
Vaccines are also monitored for safety in multiple ways after they are
released for public use. Because rare reactions, delayed reactions, or reactions
within sub-populations may not be detected before vaccines are licensed,
post-licensure (also called post-marketing) evaluation of vaccine safety when
millions of persons may be vaccinated is critical. Historically, this has relied
on passive surveillance systems like the Vaccine Adverse Event Reporting System
(VAERS) and ad hoc epidemiologic studies. More recently, Phase IV trials and
pre-established large-linked databases (LLDBs) have been added to improve our
methodology capabilities to study rare risks of specific
immunizations.15 Furthermore, because vaccines are biologic rather
than chemical in nature, variation in rates of adverse events (and
immunogenicity) by manufacturer, 26,27 or even by lot might be
expected.28 Post-licensure surveillance systems may detect potential
lot-specific irregularities in a timely manner.
Passive Surveillance (Spontaneous Reporting System)
Passive surveillance or spontaneous reporting system (SRSs) have been the
cornerstone of most vaccine safety monitoring systems because of their relative
low cost of operations.29,30 National reporting of vaccine adverse
events can be done through the same reporting channels as those used for other
adverse drug reactions.30 Vaccine manufacturers also maintain SRSs
for their products, which are usually forwarded subsequently to appropriate
national authorities.29
Due to the importance of infectious disease control, vaccines are purchased
and administered by national public health authorities. For example, the public
sector ( federal, state, and local governments) coordinates its programs with
the Centers for Disease Control and Prevention (CDC). The CDC purchases and
distributes more than half of the childhood vaccines administered in the United
States. The CDC collaborated with FDA, which licenses and regulates vaccines, in
developing the Vaccine Adverse Event Reporting System (VAERS).
The Vaccine Adverse Event Reporting System (VAERS)
VAERS is known as a passive surveillance system because it depends on health
care providers and/or patients and others to file adverse event reports. The
National Childhood Vaccine Injury Act of 1986 mandated, for the first time, that
health providers report certain adverse events after immunizations.31
VAERS was implemented jointly by the CDC and FDA in 1990 to provide a unified
national system for collection of all reports of clinically significant adverse
events, including but not limited to those mandated for reporting.1
The creation of VAERS also provided an opportunity to correct some shortcomings
of the predecessor CDC Monitoring System for Adverse Events Following
Immunizations (MSAEFI) and FDA Adverse Drug Reaction. 32
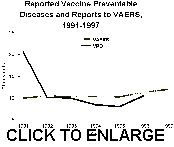 Figure 2. Reported vaccine-preventable diseases (VPD) and reports to VAERS,
1991-1997.
Figure 2. Reported vaccine-preventable diseases (VPD) and reports to VAERS,
1991-1997.
Health effects reported to VAERS as being associated with vaccines may be
either 1) true adverse reactions or 2) associated with vaccination only by
coincidence but falsely attributed to the vaccine. In the United States,
approximately 10,000 reports of both types of events are reported to VAERS each
year, with approximately 20% classified as serious.33 Professional
follow-up occurs on all reports of deaths and some selected serious events of
interest. Today, the current number of reports to VAERS exceeds the reported
incidence of most vaccine-preventable childhood diseases combined (figure 2).
Close monitoring and timely assessment of these suspected vaccine adverse events
are critical to prevent the public's loss of confidence in vaccines.
The VAERS reporting form is designed to allow a narrative description of
adverse events. All persons, including patients, parents (constitute less than
5% of VAERS reports), and health professionals, can report to VAERS. There are
no restrictions on onset intervals or requirements for medical care. Annually,
VAERS forms are sent to approximately 200,000 physicians in the specialties of
pediatrics, family practice, general practice, internal medicine,
obstetrics/gynecology, and emergency medicine. Copies are also sent to state
health departments and to public clinics that administer vaccines. Information
sought on the VAERS report include the vaccine received, the timing of the
vaccination and the onset of adverse events, demographic information about the
recipient, information about concurrent medical illnesses or medications, and
past history of adverse events after vaccination. The form is preaddressed and
postage-paid so that after completion it can be folded and mailed. To request a
VAERS form, assistance in completing the form, or answers to other questions
about the reporting system, call 1-800- 822-7967.
A contractor, under CDC and FDA supervision, distributes, collects, codes
(using the Coding Symbols for a Thesaurus of Adverse Reaction Terms) (COSTART),
and enters VAERS reports in a database. A verification-of-receipt letter,
bearing the assigned VAERS identification number is returned to the reporter.
Reporters of selected serious events receive written requests from VAERS (60
days after vaccination and one year after vaccination) for information about the
patient's recovery. Reporters may also submit additional relevant information to
the VAERS by using the assigned identification number. Both the CDC and FDA have
online computer access to the VAERS database and focus their efforts on
analytical tasks of interest to their respective agencies. These data (minus
personal identifiers) are also available to the public.
Classifications, Case Definitions and Evaluative Protocols
Vaccine reactions can be classified by frequency (common, rare), extent
(local, systemic), severity (hospitalization, disability, death), causality, and
preventability (intrinsic to vaccine, faulty production, faulty administration).
A recent classification divides adverse events after
vaccinations:34,35
1. Vaccine-induced: due to the intrinsic characteristic of the vaccine
preparation and the individual response of the vaccinee, these events would not
have occurred without vaccination (e.g., vaccine-associated paralytic
poliomyelitis).
2. Vaccine-potentiated: the event would have occurred anyway, but was
precipitated by the vaccination (e.g., first febrile seizure in a predisposed
child).
3.Programmatic error: the event occurred due to technical errors in
vaccine preparation, handling, or administration.
4. Coincidental: the event was associated temporally with vaccination
(e.g. by chance occurrence or due to underlying illness).
To further improve the quality of SRS data and maximize its utility as a
registry of rare potential vaccine reactions, standard protocols for the
clinical evaluation of selected serious events reported to VAERS (e.g., deaths,
seizures) are under development. Such protocols could then be sent to the health
care providers who report such events in order to standardize the evaluation of
these patients.
Assessment of Causality of Vaccine Adverse Events
The formal process of assessing causality of an adverse event and an exposure
(e.g., vaccine) is a complex process that can be considered in terms of the
answers to three questions: Can It?, Did It?, and Will
It?13,14,36 For individual case reports, the "Did It?"
question is more relevant. If the answer is yes, then "Can It?" is also
answered in the affirmative. It is natural to suspect the vaccine to be the
cause when an adverse event occurs following vaccination, but in reality a
causal association may or may not exist. Information used for assessing
causality in individual case reports includes the following: a) previous general
experience with vaccine; b) alternative etiologies; c) susceptibility of the
vaccinee; d) timing of events; e) characteristic of the event (e.g.,
confirmatory laboratory findings); f) dechallenge; g) rechallenge.
When a vaccine can cause an adverse event, the question "Will It?" refers to
the probability that an individual will experience the event or, for
populations, the proportion that will experience it (i.e., the attributable
risk). These data are critical for risk-benefit considerations and are generally
available only when the "Can It?" question is answered based on epidemiologic
studies? 14
Another approach to causality that minimizes controversy is to assume that
adverse events that occur within a particular period after vaccinations are
caused by the vaccine, irrespective of whether they were truly causal or just
coincidental. This approach to causality is used in some vaccine injury
compensation programs to simplify the proceedings.37 Classifications
are based on the reported symptoms, the interval between vaccination and onset
of symptoms, and a set of case definitions.
Usually, causal link between the vaccine and an adverse event can be
established if there is a unique laboratory diagnostic result (e.g., viral
culture in patient with adverse event and genetic sequencing showing virus is a
vaccine and not a wild strain);38 a unique clinical syndrome (e.g.,
acute flaccid paralysis classical for polio occurring shortly after receipt of
oral polio vaccine in setting where wild polio virus circulation is
unlikely);39 or an epidemiologic study showing vaccinated people are
more likely than unvaccinated people to experience the adverse event.
Unfortunately, very few VAERS reports meet either of the first two criteria.
 Figure 3. Establishing a causal link: adverse event and vaccine.
Figure 3. Establishing a causal link: adverse event and vaccine.
Because VAERS reports come from just vaccinated people with adverse events, they
represent just cell "a" of such a "2 x 2" table needed for an epidemiologic
study (figure 3). The information needed to complete the other 3 cells is
usually missing. This explains, in part, the relative lack of knowledge
regarding vaccine safety found by the IOM and the value LLDBs for studying
vaccine safety, because all the data to complete this table are readily
available via this approach. CDC's Vaccine Safety Datalink (VSD) project is one
example of such an LLDB. It links the immunization and medical records on
members of four HMO's, totaling 2% of the US population for various vaccine
safety studies.
Large-Linked Databases (LLDB)
Historically, when a signal of a potential vaccine safety concern was
generated from passive surveillance, ad hoc epidemiologic studies were needed to
test this hypothesis. Such ad hoc studies while, potentially informative about
vaccine causality, are costly, time-consuming, and usually limited to assessment
of a single hypothesis.
The limitations of VAERS and the recognition of the need for improved
monitoring of vaccine safety prompted the CDC to initiate the VSD project in
1990.22 To help overcome the previously identified shortcomings, the
VSD study prospective collects vaccination, medical outcome (e.g., hospital
discharge, outpatient, emergency room, deaths), and covariant data (e.g., birth
certificates, census) under joint protocol at multiple HMOs. Selection of staff
model prepaid health plans also minimized potential biases resulting from data
generated from fee-for-service claims. The VSD has conducted active surveillance
on approximately 500,000 children from birth through 6 years of age (75,000
birth cohort, approximately 2% of U.S. population in these age groups) whose
parents (or legal guardians) were enrolled in one of four staff model
HMOs.22 Expansion to include all age groups in the study is underway.
Each site encodes their patients' clinical data with unique study identifiers
before sending to data to CDC annually for merging and analysis, thereby
preserving patient confidentiality. Depending on the background incidence of the
medical event, the frequency of specific vaccinations and their relative risk,
associations of 1 per 100,000 doses should be detectable within 5 years.
The VSD has focused its initial efforts on examining potential associations
between immunizations and 34 serious neurologic, allergic, hematologic,
infectious, inflammatory, and metabolic conditions. The VSD is also being used
to test new ad hoc vaccine safety hypotheses. These may arise from the medical
literature,40 from VAERS,27 from changes in immunization
schedules,41 or from the introduction of new vaccines.42
The diversity in vaccination practice at the four HMOs and the clinic-to-clinic
and day-to-day variations in practice permit useful contrasts in safety
experiences.41 The size of the VSD population may also permit
separation of the risks associated with individual vaccines from those
associated with vaccine combinations, whether given in the same syringe or
simultaneously at different body sites. Such studies will be especially valuable
in view of the new combined pediatric vaccines currently in
development.43
Should the VSD study identify a vaccine reaction, data on attributable risk
will be available, thereby permitting accurate risk-benefit assessment by both
the public and policymakers.44 Subgroup analyses may permit
identification of risk factors, which may be useful in identifying
contraindications to vaccinations. The incidence rates of reactions identified
in VSD should permit the evaluation and improvement of passive surveillance
systems like VAERS. The VSD data can also aid the FDA27 and the
Vaccine Injury Compensation Program.45
Amid these promises, a few caveats are appropriate. While diverse, the
population in the four HMOs currently in the VSD is not wholly representative of
the United States in terms of geography or socioeconomic status. More
importantly, due to the high coverage attained in the HMOs for most vaccines,
few nonvaccinated controls are available. The VSD must, therefore, rely
predominantly on some type of "risk-interval" analysis.46,47 The
capability of this approach to assess associations between vaccination and
adverse events with delayed or insidious onset (e.g. autism) is limited.
Similarly, the ability of the VSD to fully distinguish effects of combined or
simultaneous vaccination may be limited should such practices become universal.
The VSD also cannot easily assess adverse events not currently captured in
existing HMO databases, either because they do not result in a health care
consultation or because the data are not automated.22 The patient
enrollment, health care practices, and health information systems at each HMO
are dynamic, which may either aid or impede study of specific outcomes. Coding
error occurs inevitably in all data files to some extent, resulting in a
decrease in our ability to detect a true association. The current VSD is also
unable to examine the risk of extremely rare events after infrequent
vaccinations, such as Guillain-Barré syndrome, after each season's flu vaccine.
Because the VSD relies on epidemiologic methods, it may not successfully control
for confounding and bias in each analysis48 and inferences on
causality may be limited.
Despite these potential shortcomings, the VSD provides a new, essential,
powerful, and cost-effective complement to our ongoing evaluations of vaccine
safety in the United States. In view of the methodologic and logistical
advantages offered by LLDBs, the United Kingdom and Canada have also developed
LLDBs, linking immunization registries with medical files.18,21
Because of the relatively limited number of vaccines used worldwide and the
costs associated with establishing and operating them, it is unlikely that all
countries will be able to or need to establish their own LLDBs. They should be
able to draw upon the scientific base established by the existing LLDBs for
vaccine safety and, if needed, to conduct ad hoc epidemiologic studies.
Vaccine Administration Considerations
In order to maintain their proper biologic activity, vaccines must be
properly shipped, stored, and administered. Failure to adhere to these
requirements can lead to loss of vaccine potency, resulting in an inadequate
immune response to the vaccine.49 Proper storage and handling
requirements for each vaccine are given on the manufacturer's information
insert. This information can also be found in recommendations of the
Advisory Committee on Immunization Practices of the CDC and the American Academy
of Pediatrics. 50-52 Another concern is the growing complexity of
vaccines (table 1), including the growing variety of multiple combination
vaccines that clinics will be faced with keeping in clinic stock.53
These decisions will become more challenging, especially when patients change
health care providers and the providers stock different vaccine combinations. To
prevent administration errors, adequate and accurate recording of combination
vaccines in patient charts will become increasingly important to ensure proper
practices are followed. Immunization staff will continue to need education in
appropriate vaccine storage, handling, and safe injection practices.
54
In developing countries, vaccine safety concerns frequently relate more to
inadequate control of vaccine production and administration errors such as reuse
of needles resulting in the transmission of blood-borne pathogens. It is
anticipated, however, that as nations in both developed and developing countries
reach high vaccine coverage and vaccine-preventable diseases become less
visible, public concerns about vaccine safety may also threaten the stability of
current immunization programs.3
Vaccine Injury Compensation Program
Because many vaccinations are mandated for public health reasons and because
no vaccine is perfectly safe, several countries have established compensation
programs for people who may have been injured by vaccination.37
During the 1980's in the United States, increases in reports about adverse
events following vaccination led to an increased number of lawsuits against
vaccine manufacturers.55 As a result, substantial increases in the
price of vaccines began to occur as well as a loss of manufacturers willing to
produce vaccines.56 Due to these disturbing trends, the National
Childhood Vaccine Injury act of 1986 established the National Vaccine Injury
Compensation Program. This program was designed to compensate those individuals
or families of individuals who have been injured by childhood vaccines on a
"no-fault" basis, based on a predetermined Vaccine Injury Table. Accurate
assessments of whether adverse events can be caused by specific vaccines are
required to update the Vaccine Injury Table as to justly compensate people
injured by true reactions and to disallow false or unrelated claims.45
The program is administered jointly by the Department of Health and Human
Services and the Department of Justice. To obtain an information packet on how
to file a claim, criteria for eligibility, and documentation required, call
1-800-338-2382 or write to the National Vaccine Injury Compensation Program,
Parklawn Building, Room 8A-35, 5600 Fishers Lane, Rockville, Maryland 20857.
Vaccine Risk Communication Considerations
Almost every child in this country is vaccinated, and therefore almost every
parent has a question about vaccines. Parents want to know what vaccines are
made of, if they work, and whether they are safe.57 Inaccurate or
misleading information relating to vaccines is readily available to parents and
can be found on the Internet and in some publications. The art of handling
vaccine safety concerns and vaccine risk communication has emerged as an
increasingly important skill for health care providers. Periodically, vaccine
safety concerns also emerge in the media. The media aims to present both sides
of a story and not judge the strength of one side over another. The challenge
for healthcare professionals is to establish greater credibility with the
audience in instances of inaccurate or misleading news reports.58,59
Health Risk communication has traditionally consisted of messages designed to
encourage behavior that reduces individual and societal risk (e.g., smoking
cessation).59 Risk communication is seen as an interactive process of
an exchange of information and opinion among individuals, groups, and
institutions.62 Risk communication, while having a 20 year history as
a field of study, is still an emerging field in vaccine education. A big
challenge in vaccine risk communication is acknowledging and defining
uncertainty about the very small estimates of risk associated with vaccination.
Risk communication is more effective when this uncertainty is stated and when
risks are quantified as much as science permits. Trust is a key component of the
exchange of information at every level, and overconfidence about risk estimates
that are later shown to be incorrect contribute to a breakdown of trust among
people involved.59,61 Disease prevention is a difficult task,
especially when it requires participation by a majority of the population. In
the pre-immunization era, vaccine-preventable diseases like measles and
pertussis were so widespread that the risks and benefits of disease compared
with vaccination were readily evident. As immunization programs successfully
reduced the incidence of disease, an increasing number of health care providers
and parents have had little or no personal experience with these once common
diseases. These individuals are forced to rely on history and other more
"distant" descriptions of these diseases in textbooks or educational brochures.
In contrast, what is visible is the degree of very personal discomfort
associated with each immunization.
Principles of Risk Communication
 Some key principles and lessons can be applied to vaccine risk communication:
1) Individuals differ in their perceptions of risk depending on their life
experience and knowledge; 2) Certain risks are more acceptable to people than
other risks (table 2); and 3) Risk communication is an interactive process that
requires active listening and discussion.
Some key principles and lessons can be applied to vaccine risk communication:
1) Individuals differ in their perceptions of risk depending on their life
experience and knowledge; 2) Certain risks are more acceptable to people than
other risks (table 2); and 3) Risk communication is an interactive process that
requires active listening and discussion.
People who have had an adverse reaction or event following vaccination or
know someone who has had a bad experience may perceive vaccinations as being
more risky than those who have not. On the other hand, patients who have had a
vaccine-preventable disease, like polio, and physicians who worked in polio
wards in the 1950s are more likely to be stronger advocates of polio
vaccination than patients or physicians who never saw wild polio disease.
Personal stories can be a powerful influence and motivator, especially if they
are emotional compelling.
In an analysis of Internet web pages of parent groups with concerns about
vaccination, personal stories about adverse reactions believed to be associated
with vaccines were one of the most frequently highlighted features. 62
Knowledge can also be a powerful influence in risk decisions. People may
perceive a greater risk for things that they do not know about versus those
things with which they are familiar. For instance, Measles vaccine may be
perceived as less risky than a vaccine that sounds unfamiliar and exotic, such
as Hib.
Identifying the source of a parent's information (e.g. news stories,
alternative health, vaccine information statements, and office staff,) can help
focus your interaction and is an important first step in the risk communication
process. In informal discussions with parents who had concerns about vaccines at
a recent parent conference focusing on vaccine safety, sources of parent
information included a wide range of materials, including nontraditional health
resources, which they perceived as being as credible as information from
traditional sources. The perception of having knowledge to control the outcome
of whether one's children become infected with vaccine-preventable diseases also
affects the acceptance of vaccine risks. In one study, nonvaccinators believed
they could prevent their children from catching whooping cough, and they also
thought it was less likely that their child would be disabled or killed by
disease in the absence of vaccination.63 Understanding a patient's
source of information provides a key to an effective approach in correcting
misconceptions about vaccines. In some cases, source correction, in addition to
patient counseling, should be considered (for instance, writing a letter to the
editor of a recent inaccurate news publication).
Another consideration in assessing knowledge is to recognize a person's
learning style. One style used by people is to seek out a trusted source for
information on vaccines; and for many people, this is their pediatrician.
Another style is to seek out many different sources of information. Persons
using this style will actively look for several different resources to confirm
or expand their perceptions about a topic. In an analysis of calls coming into
the National Immunization Hotline, several callers were confirming vaccine
information that their physician had provided them with hotline staff. For some
people, these confirmation behaviors become more pronounced in risk/benefit
decisions. The more risky the perception, the greater the need to seek multiple
opinions about the perceived risky behavior. Providing these people with several
different resources they can go to in their learning process may be a helpful
intervention (see the vaccine safety resource appendix for helpful references
and resources that can be shared with patients).
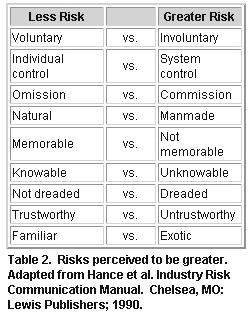
Risk perceptions are individual; however, some risk are naturally more
acceptable to people than others.64 The risk perception comparisons
seen in figure 9 have been adapted from risk perceptions as
they relate to environmental concerns, but can be applied to immunization
practices as well. A discussion on a few of these and how they relate follows.
Voluntary risks are usually more acceptable than involuntary risks. For most
people, assuming the risk of a vaccine side effect after a free, voluntary,
company-offered, flu vaccine program is more acceptable than assuming the risk
of a side effect from a company-required flu immunization program.
Many state laws require vaccination upon school entry (system control),
which can be perceived as more risky. Most of these states also offer religious
and philosophical exemptions to vaccination, allowing for less risk (individual
control).
For two equal risks, one due to an action and one due to inaction, most
people prefer the consequences of inaction.63 That is to say, if
there were an equal risk of a bad outcome from an action (such as an adverse
reaction to vaccination) and inaction (such as getting a disease due to
nonvaccination), many people have an "omission bias": they would prefer the
consequences of omission to commission.
In the United States, information developed by the CDC about the risks and
benefits of immunizations is required to be provided to all public sector people
receiving vaccinations since 1978.65 Recent efforts have been devoted
to the use of focus groups and other research to assess and improve the
effectiveness of such information material. Studies on the use of positive
versus negative framing to help people put risks in perspective have had good
results.66,67 An example of positive framing is presenting the
percentage of people who have no adverse reactions to vaccines instead of
framing the statement negatively as the percentage of those who do. In many
countries, including the United States, people who believe that their children
or themselves have been injured by vaccines have organized to distribute through
the Internet and various publications information that highlights the risks of
and uncertainties related to immunizations. Materials to address these
misconceptions and allegations about immunizations are developed by the National
Immunization Program and distributed to state immunization programs as they
arise. These materials can be obtained from the National Immunization Hotline
(800) 232-2522 and the National Immunization Program Web Page (www.cdc.gov/nip).
In the future, we will need to create better systems to assess concerns, develop
and disseminate materials to address public needs, and develop present day and
historically based materials that can express the impact of outbreaks of
vaccine-preventable diseases.
Risk communication can be used for the purposes of either advocacy, public
education, or decision-making partnership. People care not only about the
magnitude of the risk, but also how the risks are managed and whether they
participate in the risk-management process, especially in a democratic
society.59,68 Immunization is unlike most other medical procedures,
in that the consequences of the decision affect not only the individual but also
others in the society. Because of this important distinction, many countries
have enacted public health (e.g., immunization) laws that severely limit any
individual's right to infect others. Some people may attempt to avoid the risks
of vaccination while being protected by the group also known as herd immunity,
which results from others being vaccinated. The protection provided by herd
immunity may disappear if too many people avoid vaccination, which may result in
tragic outbreaks of diseases. 3,69 Recent debates in the United
States have focused on whether philosophical (in addition to medical and
religious) exemptions to mandatory immunizations should be allowed more
universally and, if so, what standards for claim of exemption are needed.
Vaccine risk communication should discuss the risks and benefits of specific
vaccines and should also inform people receiving vaccinations about the delicate
balance between societal and individual rights in a shared community.
Evaluating and Managing Vaccine Safety Concerns
Empathy, patience, and a careful assessment are all needed to effectively
address vaccine safety concerns from a clinical perspective. As with all
investigations, the first step is objective and comprehensive data gathering
with an open mind. Premature dismissal of a new vaccine safety concern as
"unfounded" without gathering and weighing the evidence is unwise and
unscientific. On the other hand, endorsement of isolated findings or unproven
theories about vaccines without careful consideration to the weight of evidence
can be equally problematic.
Establishing trust and credibility are key in promoting a positive
relationship. Factors that aid in such credibility include careful assessment of
the patient's concern, scientific and medical expertise, empathy, and the
ability to distill scientific facts and figures down to easily understood
concepts. Emotionally compelling first-hand accounts of the effects of
vaccine-preventable diseases may be needed to effectively illustrate the
importance of vaccination practices.
Clarifying the distinction between theory, fact and perceived and real risk
for the concerned public is critical. What is certain is the risk associated
with the vaccine-preventable disease should a person remain non-immune. What is
also certain is the small magnitude of risk for any severe vaccine reactions.
The significance of uncertainty in vaccine safety is related to individual
perceptions. Learning how to effectively communicate with patients will become
an increasingly important skill for health care providers in ensuring the
protection of our children from vaccine- preventable diseases.
References
1. Chen RT, Rastogi SC, Mullen JR, et al. The Vaccine Adverse Event Reporting
System (VAERS). Vaccine. 1994;12:542-550
2. Duclos P.Surveillance des effets secondaires de la vaccination. Sante
1994;4:215-20.
3. Gangarosa EJ, Galazka AM, Wolfe CR, Chen RT, Phillips LM, Gangarosa, RE,
Miller E. Impact of the anti-whole-cell pertussis vaccine movements. Lancet
1997;351:356-361.
4.Galazka AM, Robertson SE, Oblapenko GP. Resurgence of diphtheria. Eur J
Epidemiol. 1995;11:95-105.
5. Wilson GS. The hazards of immunization. London: Athlone Press, 1967.
6. Schumacher W: Legal/ethical aspects of vaccinations. Develop biol Standard
1979; 43:435-8.
7. Pennisi E. Monkey virus DNA found in rare human cancers. Science
1997;275:748-9.
8. Food and Drug Administration. Bovine-derived materials: Agency letters to
manufacturers of FDA-regulated products. Federal Register 1994;59:44591-4.
9. Mathieu M, ed. Biologic development: a regulatory overview. Waltham MA:
Paraxel, 1993.
10. Neustadt RE, Fineberg HV: The swine flu affair: decision-making on a
slippery disease. Washington DC: US Government Printing Office, 1978.
11. Lloyd JC, Chen, RT. The Urabe mumps vaccine: lessons in adverse event
surveillance and response. Pharmacoepidemiology and Drug Safety. 1996; 5:S45.
Abstract.
12. Kimura M, Kuno-Sakai H, Yamazaki S et al. Adverse events associated with
MMR vaccines in Japan. Acta Paediatrica Japonica 1996;38:205-11.
13. Howson CP, Howe CJ, Fineberg HV, eds. Institute of Medicine. Adverse
effects of pertussis and rubella vaccines: A report of the Committee to Review
the Adverse Consequences of Pertussis and Rubella Vaccines. Washington, D.C.:
National Academy Press; 1991.
14. Stratton KR, Howe CJ, Johnston RB, eds. Adverse Events Associated with
Childhood Vaccines: Evidence Bearing on Causality. Washington D.C.:National
Academy Press; 1994.
15. Chen RT. Special methodological issues in pharmacoepidemiology studies of
vaccine safety. In: Strom BL, ed. Pharmacoepidemiology. Sussex: John Wiley &
Sons, 1994:581-94.
16. Fine PEM, Chen RT. Confounding in studies of adverse reactions to
vaccines. Am J Epidemiol. 1992;136:121-135.
17. Rothman KJ, ed. Causal inference. Chestnut Hill, MA: Epidemiology
Resources, Inc; 1988.
18. Farrington CP, Pugh S, Colville A, et al. A new method for active
surveillance of adverse events from diphtheria/tetanus/pertussis and
measles/mumps/rubella vaccines. Lancet. 1995;345:567-569.
19. Ellenberg SS, Chen RT. The complicated task of monitoring vaccine safety.
Public Health Reports 1997;112:10-20.
20. Morris R, Halperin S, Dery P et al. IMPACT monitoring network: a better
mousetrap. Can J Infect Dis 1993;4:194-5.
21. Roberts JD, Roos LL, Poffenroth LA, et al. Surveillance of
vaccine-related adverse events in the first year of life: A Manitoba cohort
study. J Clin Epidemiol. 1996;49:51-58.
22. Chen RT, Glasser J, Rhodes P, et al. The Vaccine Safety Datalink Project:
A New Tool for Improving Vaccine Safety Monitoring in the United States.
Pediatrics 1997;99:765-73.
23. Rosenthal KL, McVittie LD. The clinical
testing of preventive vaccines. In: Mathieu M, ed. Biologic development: a
regulatory overview. Waltham MA: Paraxel, 1993:119-30.
24. Pinichiero ME. Acellular pertussis vaccines: towards an improved safety
profile. Drug experience 1996;15:311-24.
25. Global Programme on Vaccines and Immunization. Quality: the first
consideration. Geneva: WHO/GPV/SAGE.97/WP.03.
26. Steinhoff MC, Reed GF, Decker MD, et al. A randomized comparison of
reactogenicity and immunogenicity of two whole-cell pertussis
vaccines.Pediatrics 1995 S567-70.
27. Niu MT, Rhodes P, Salive M, Lively T, Davis DM, Black S et al.
Comparative safety of two recombinant hepatitis b vaccines in children: data
from the Vaccine Adverse Event Reporting System (VAERS) and Vaccine Safety
Datalink (VSD). J Clin Epidemiol. 1998; in press.
28. Baraff LJ, Cody CL, Cherry JD: DTP-associated reactions: an analysis by
injection site, manufacturer, prior reactions, and dose. Pediatrics 1984;
73:31-6.
29. Rastogi SC ed. International Workshop:Harmonization of Reporting of
Adverse Events Following Vaccination. Rockville MD: FDA/CBER, 1993.
30. Wiholm BE, Olsoon S, Moore N, Wood S. Spontaneous reporting systems
outside the U.S. In: Strom BL, ed. Pharmacoepidemiology. Sussex: John Wiley
& Sons, 1994:139-55..
31. Centers for Disease Control. National Childhood Vaccine Injury Act:
Requirements for Permanent Vaccination Records and for Reporting of Selected
Events after Vaccination. MMWR 1988;37:197-200.
32. Stetler HC, Mullen JR, Brennan JP, Livengood JR, Orenstein WA, Hinman AR.
Monitoring System for Adverse Events Following Immunization. Vaccine.
1987;5:169-174.
33. Braun MM, Ellenberg SS. Descriptive epidemiology of adverse events
following immunization: reports to the Vaccine Adverse Event Reporting System
(VAERS), 1991-1994. J Pediatr 1997; in press.
34. Fenichel GM, Lane DA, Livengood JR, Horwitz SJ, Menkes JH, Schwartz JF:
Adverse events following immunization: assessing probability of causation.
Pediatr Neurol 1989; 5:287-90.
35. Wassilak SGF, Sokhey J: Monitoring of adverse events following
immunization in the Expanded Programme on Immunization. World Health
Organization 1991; WHO/EPI/GEN/91.2:1-29.
36. Kramer MS, Lane DA. Causal propositions in clinical research and
practice. J Clin Epidemiol 1992; 45:639-49.
37. Mariner WK: Compensation programs for vaccine-related injury abroad: a
comparative analysis. Saint Louis University Law Journal 1987; 31:599-654.
38. Forsey T, Mawn JA, Yates PJ, Bently ML, Minor PD. Differentiation of
vaccine and wild mumps viruses using the polymerase chain reaction and
dideoxinucleotide. J Gen Virol 1990;71:987-90.
39. Henderson DA, Witte JJ, Morris L, Langmuir AD. Paralytic disease
associated with oral polio vaccines. JAMA 1964;190:153-60.
40. Ray WA, Griffin MR. Confounding in studies of adverse reactions to
vaccines (letter). Am J Epidemiol 1994;139:229.
41. Davis R, Vadheim C,
Black S, et al. Immunization tracking systems: experience of the CDC Vaccine
Safety Datalink sites. HMO Practice 1997;11:13-7.
42. Black S.B., Shinefield H.R., and the Northern California Permenente
Medical Care Program Department of Pediatrics Vaccine Study Group. b-CAPSA I
Haemophilus influenzae, typeb capsular polysaccharide vaccine safety. Pediatrics
1987;79:321- 325.
43. Williams JC, Goldenthal KL, Burns DL, Lewis BP Jr, eds. Combined vaccines
and simultaneous administration: current issues and perspective. New York: New
York Academy of Science; 1995.
44. Hinman AR, Orenstein WA. Public health considerations. In: Plotkin SA,
Mortimer EA, eds. Vaccines. Philadelphia: WB Saunders; 1994: 903-932.
45. Evans G. Vaccine liability and safety: a progress report. Pediatr Infect
Dis J 1996:15:477-8.
46. Walker AM, Jick H, Perera DR, et al. Neurologic events following
diphtheria-tetanus-pertussis immunization. Pediatrics. 1988;81:345-349.
47. Griffin MR, Ray WA, Mortimer EA, et al. Risk of seizures and
encephalopathy after immunization with the diphtheria-tetanus-pertussis vaccine.
JAMA. 1990;263:1641-1645.
48. Fine PEM. Methodological issues in the evaluation and monitoring of
vaccine safety. Ann NY Acad Sci 1995; 754:300-8.
49. Watson JC, Peter G. General Immunization Practices. In: Plotkin SA,
Orienstein WA (eds). Vaccines 3rd edition. WB Saunderes,
Philladelphia, 1998. (In Press).
50. American Academy of Pediatrics. Active immunization. In: Peter G, ed 1997
Redbook:Report of the Committee on Infectious Diseases. 24th ed. Elk
Grove Village, IL:American Academy of Pediatrics;1997:4-36.
51. U.S. Department of Health and Human Services, Public Health Service, CDC.
Vaccine management recommendations for handling and storage of selected
biologicals. March 1991.
52. U.S. Department of Health and Human Services, Public Health service, CDC.
Guidelines for vaccine packing and shipping. January 1997.
53. Weniger BG, Chen RT, Jacobsen SH, Sewell EC, Dueson R, Livengood JR, et
al. Addressing the challenges to immunization practice with an economic
algorithm for vaccine selection. Vaccine, 1998, in press.
54. Zaffran M, Lloyd J, Clements J, Stilwell. A drive to safer injections.
Geneva: WHO/GPV/SAGE.97/WP.05, 1997.
55. Orenstein WA. DTP vaccine litigation, 1988. Am J Dis Child 1990;144:517.
56. Institute of Medicine: Liability for the production and sale of vaccine.
In: Vaccine supply and innovation. Wash D.C.: National Academy Press, 1985;
85-122.
57. Offit PM, Bell LM. What every parent should know about vaccines. Simon
&Schuster Macmillan company. New York, NY 1998:1.
58. Freed GL, Katz SL, Clark SJ. Safety of vaccinations: miss American, the
media, and public health. JAMA. 1996;276:1869-1872, 1917-1918.
59. Institute of Medicine: Risk Communication and Vaccination, Workshop
summary. Wash D.C.: National Academy Press, 1997.
60. National Research Council. Improving risk communication. Washington DC:
National Academy Press, 1989.
61. Fischhoff B, Bostrom A, Jacobs-Quadrel M. Risk perceptions and
communcation. Annual Review of Public Health 1993;14:182-203
62. Hibbs
BF, Wolfe S, Chen RT. Internet Web page perceptions of vaccine
pharmacoepidemiology research: an analysis of vaccine safety concerns. Abstract
129. Abstracts of the 13th International Conference on
Pharacoepidemiology. Walt Disney World, Florida: August 24-27, 1997.
Pharmacoepidemiology and Drug Safety. 1997; 6 (suppl 2): S60.
63. Meszaros JR, Asch DA, Baron J, Hershey JC, Kunreuther H, Schwartz-Buzaglo
J. Cognitive processes and the decisions of some parents to forego pertussis
vaccination for their children, Journal of Clinical Epidemiology
1996;49:697-703.
64. Hance BJ, Chess C, Sandman P. Industry risk communication manual.
Chelsea, MI. Lewis Publishers 1990.
65. The National Childhood Vaccine Injury Act of 1986, at Section 2125 of the
Public Health Service Act as codified at 42 U.S.C. § 300aa-(Supp.1987).
66. Tversky A, Kahneman D. Availability:A Heuristic for judging frequencey
and probability. Cognitive Psychology 1973;5:207-232.
67. Baron J. The effect of normative beliefs on anticipated emotions. Journal
of Personality and Social Psychology 1992;63:320-330.
68. Bostrom A. Vaccine Risk Communication:Lessons from Risk Perception,
Decision making and Environmental Risk Communication Research. 8 Risk: Health,
Safety&Environment 173: Spring 1997:173-200.
69. Binkin NJ et al. Epidemiology of Pertussis in a developed country with
low vaccination coverate:the Italian Experience. 1992. Pediatr. Infect. Dis. J.
11:653.
 Some key principles and lessons can be applied to vaccine risk communication:
1) Individuals differ in their perceptions of risk depending on their life
experience and knowledge; 2) Certain risks are more acceptable to people than
other risks (table 2); and 3) Risk communication is an interactive process that
requires active listening and discussion.
Some key principles and lessons can be applied to vaccine risk communication:
1) Individuals differ in their perceptions of risk depending on their life
experience and knowledge; 2) Certain risks are more acceptable to people than
other risks (table 2); and 3) Risk communication is an interactive process that
requires active listening and discussion.  Figure 1. Evolution of immunization program and prominence of vaccine safety.
Figure 1. Evolution of immunization program and prominence of vaccine safety. Figure 2. Reported vaccine-preventable diseases (VPD) and reports to VAERS,
1991-1997.
Figure 2. Reported vaccine-preventable diseases (VPD) and reports to VAERS,
1991-1997. Figure 3. Establishing a causal link: adverse event and vaccine.
Figure 3. Establishing a causal link: adverse event and vaccine.