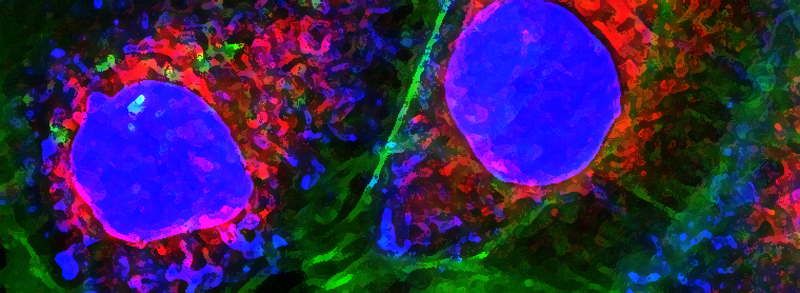
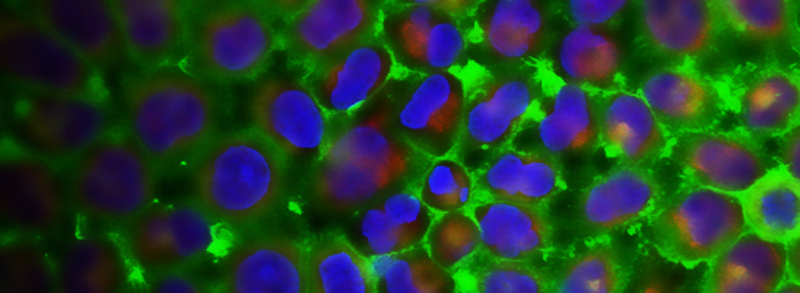
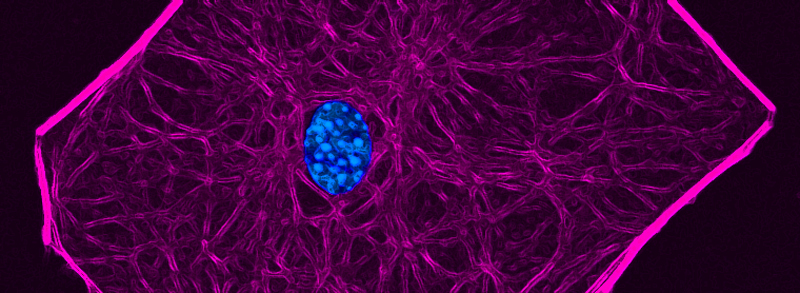
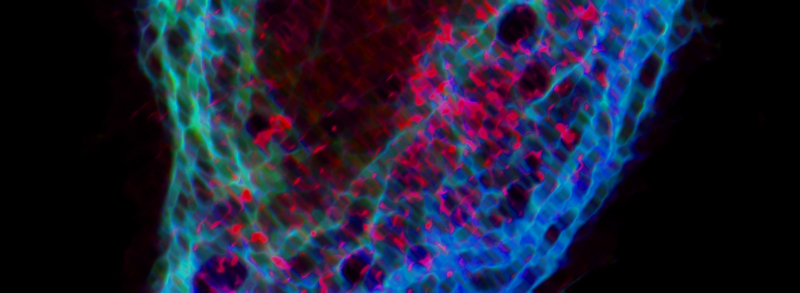

RECENT PUBLICATIONS
Loss of Tankyrase-mediated destruction of 3BP2 is the underlying pathogenic mechanism of cherubism.
Levaot N, Voytyuk O, Dimitriou I, Sircoulomb F, Chandrakumar A, Deckert M, Krzyzanowski PM, Scotter A, Gu S, Janmohamed S, Cong F, Simoncic PD, Ueki Y, La Rose J, Rottapel R.
Cell. 2011 Dec 9;147(6):1324-39.
Cherubism is an autosomal-dominant syndrome characterized by inflammatory destructive bony lesions resulting in symmetrical deformities of the facial bones. Cherubism is caused by mutations in Sh3bp2, the gene that encodes the adaptor protein 3BP2. Most identified mutations in 3BP2 lie within the peptide sequence RSPPDG. A mouse model of cherubism develops hyperactive bone-remodeling osteoclasts and systemic inflammation characterized by expansion of the myelomonocytic lineage. The mechanism by which cherubism mutations alter 3BP2 function has remained obscure. Here we show that Tankyrase, a member of the poly(ADP-ribose)polymerase (PARP) family, regulates 3BP2 stability through ADP-ribosylation and subsequent ubiquitylation by the E3-ubiquitin ligase RNF146 in osteoclasts. Cherubism mutations uncouple 3BP2 from Tankyrase-mediated protein destruction, which results in its stabilization and subsequent hyperactivation of the SRC, SYK, and VAV signaling pathways.
