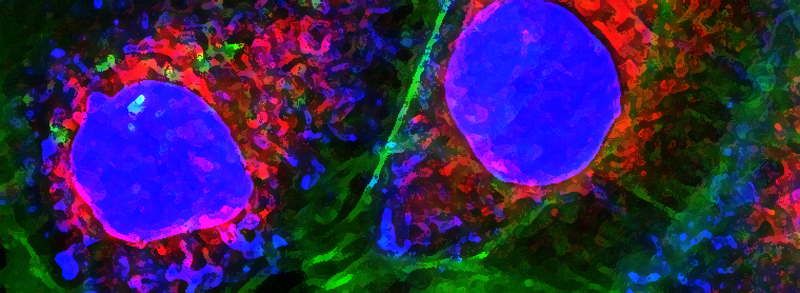
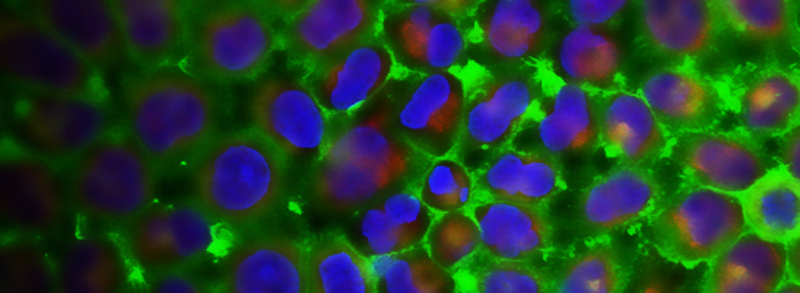
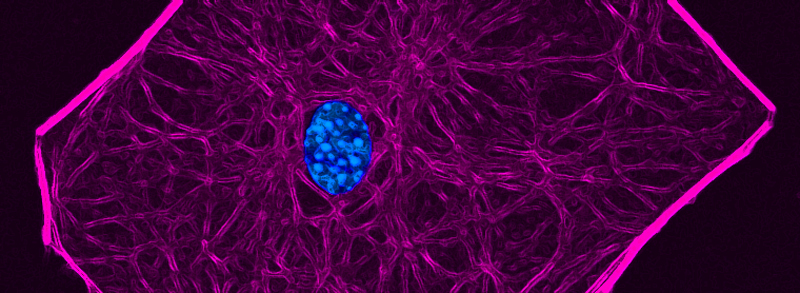
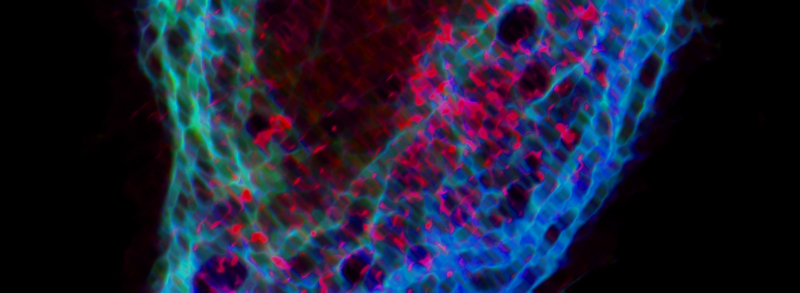

RECENT PUBLICATIONS
MARK3-mediated phosphorylation of ARHGEF2 couples microtubules to the actin cytoskeleton to establish cell polarity.
Sandí MJ, Marshall CB, Balan M1, Coyaud É, Zhou M, Monson DM, Ishiyama N, Chandrakumar AA, La Rose J, Couzens AL, Gingras AC, Raught B, Xu W, Ikura M, Morrison DK, Rottapel R.
Sci Signal. 2017 Oct 31;10(503). pii: eaan3286. doi: 10.1126/scisignal.aan3286.
The PAR-1-MARK pathway controls cell polarity through the phosphorylation of microtubule-associated proteins. Rho-Rac guanine nucleotide exchange factor 2 (ARHGEF2), which activates Ras homolog family member A (RHOA), is anchored to the microtubule network and sequestered in an inhibited state through binding to dynein light chain Tctex-1 type 1 (DYNLT1). We showed in mammalian cells that liver kinase B1 (LKB1) activated the microtubule affinity-regulating kinase 3 (MARK3), which in turn phosphorylated ARHGEF2 at Ser151 This modification disrupted the interaction between ARHGEF2 and DYNLT1 by generating a 14-3-3 binding site in ARHGEF2, thus causing ARHGEF2 to dissociate from microtubules. Phosphorylation of ARHGEF2 by MARK3 stimulated RHOA activation and the formation of stress fibers and focal adhesions, and was required for organized cellular architecture in three-dimensional culture. Protein phosphatase 2A (PP2A) dephosphorylated Ser151 in ARHGEF2 to restore the inhibited state. Thus, we have identified a regulatory switch controlled by MARK3 that couples microtubules to the actin cytoskeleton to establish epithelial cell polarity through ARHGEF2.
