Research
Placental dysfunctions of different etiologies affect 5-10% of all pregnancies and have few diagnostic markers and treatment options, resulting in poor maternal and foetal outcomes. Why is this? There are complexities in the disease that are unappreciated and which require new hypothesizes and a different technical approach.
Beyond this there are also social complications. Women’s health research was traditionally underfunded. This has changed dramatically in the last few years but we have a long way to catch up. Culturally, some women feel a sense of shame with the loss of a pregnancy and often suffer silently. Infertility has become a privatized medicine in Canada and many other parts of the world. Removing infertile couples from public health care has taken pressure off the government from funding researchers working on this area.
We work on this area because there are a large number of women and babies out there who need better health care. Many chronic health issues may have their origin in the womb or early in development. Diseases like pre-eclampsia affect the long term health of mothers and their children. A healthier future begins with a healthy pregnancy and birth.
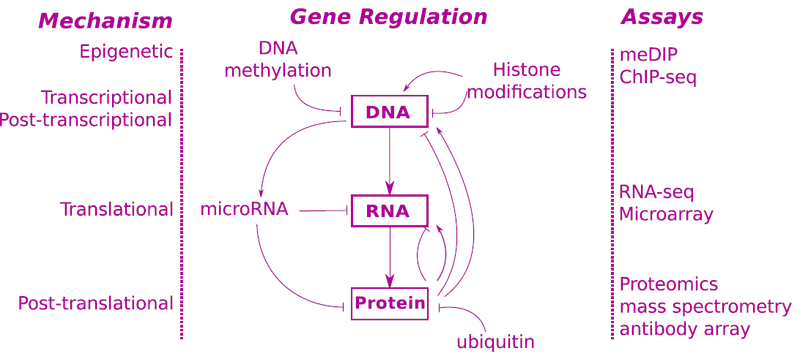
A schematic of a system biologist's view of the central dogma of biology. Multiple layers of feedback mechanisms can regulate gene expression post-transcription (microRNAs) or post-translation (ubiquitin).
On the left are the regulatory mechanisms that affect the expression of a gene from DNA to protein and on the right are different assays used to measure effects of regulatory mechanisms. (Image Brian Cox, unpublished)
Text at left links to relavant published papers listed on the Publications page.
The Cox Systems Biology Lab is engaged in a program of research on human trophoblast and the placenta. We are exploring the changes in gene regulation and cell fate that affect development and release of endocrine signals. We assemble genome wide data sets to build systems biology models of development and disease. We use a novel combination of cell sorting, surface proteomics and genome-wide expression analysis of protein, mRNA and microRNAs. These models will identify the different etiologies of complex diseases of pregnancy, as well as identify new diagnostic markers and treatments, resulting in healthier mothers and children.
Back to topOur current research program is centred around three questions:
Question 1
How is the placenta developed and organized?
Our goals are to characterize the different cell types that make up the placenta, the timing of their origins and gene expression specific to each cell type. This will lead to a better understanding of the cellular organization of the placenta and the functional roles of the different cell types. It will also identify the cell-type specific changes in populations and gene expression that occur in placental pathologies, enabling a targeted approach to disease classification and development of therapies.
Question 2
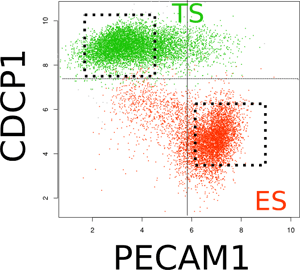
A flow cytometry plot of the separation of trophoblast stem cells (TS, green) and embryonic stem cells (ES, red) using the cell surface proteins CDCP1 (trophoblast stem cell marker) and PECAM1 (embryonic stem cell marker).
This analysis enables the separation of different cell fates from a mixture of cells that are differentiating. Black boxes are the gates that are used to isolate the different populations of cells from a mixture (Image Brian Cox, unpublished).
What are the genetic origins of the human placenta?
Our goal is to understand the mammalian conserved and human specific origins of the trophoblast. This is will be accomplished by identifying genes essential to early trophoblast fate specification and maintenance. A better molecular understanding of the early trophoblast will be used to identify the early origins of placental disease and develop novel therapeutics and birth controls.
Question 3
How is maternal-foetal communication integrated by the placenta?
Our goal is to identify the changes in secreted small molecules and proteins during pregnancy and disease, which will directly lead to the development of novel mechanistically based intervention strategies. The different phases of foetal growth require changes in maternal physiology. The placenta, acting as the primary barrier between the maternal and foetal blood supply, is required to integrate the signals of the foetus and mother and respond with signals of its own. The range of signals produced by the placenta and how they are regulated in response to signals received from the mother or foetus is not known.
Back to top